Abstract
Background Tyrosine kinase inhibitors (TKIs) revolutionized treatment of chronic myeloid leukemia (CML). However, the first months of therapy are crucial, as optimal response is defined as the achievement of molecular milestones at 3, 6 and 12 months (mo.) and as many toxicities, also causing a TKI switch, are more frequent in the 1st year.
Methods To evaluate achievement of early molecular response (MR) and incidence of events leading to a TKI change during the 1st year of therapy, we retrospectively studied 1650 CP-CML patients diagnosed from 2012 and 2019 at 31 Hematology Centres and treated with frontline imatinib (IM) or second-generation (2G) TKIs dasatinib or nilotinib. Optimal MR at 3, 6 and 12 mo. were assessed according to 2020 ELN recommendations.
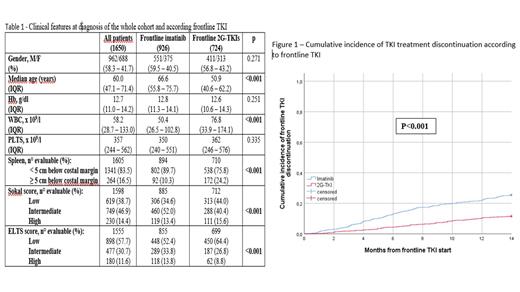
Results Frontline TKI was IM in 926 (56.1%) and 2G-TKIs in 724 (43.9%) cases: the main clinical features at diagnosis of the entire cohort and according to frontline treatment is reported in the Table 1. Commonest comorbidities were arterial hypertension (38.7%), previous neoplasm (13.6%), diabetes (11.3%), peripheral vascular diseases (7.8%), COPD (7.5%) and ischemic heart disease (6.8%). IM-treated patients were older (p<0.001), with higher ELTS score (int/high 47.6% vs 35.6%, p<0.001) and more comorbidities (p<0.005 for all diseases).
Optimal MR was achieved at 3 mo. by 1186/1430 (82.9%), at 6 mo. by 1025/1352 (75.8%) and at 12 mo. by 826/1264 patients (65.3%), respectively.
Total number of patients discontinuing TKI in the 1st year was 321/1650 (19.4%), being higher with IM (237/926, 25.5%) than 2G-TKIs (84/724, 11.6%) (p<0.001). Main causes were primary resistance (8.7%, 12.3% IM vs 4.2% 2G-TKIs, p<0.001), extra-hematologic toxicity (6.4%, 8.2% IM vs 4.2% 2G-TKIs, p>0.001), hematologic toxicity (1.7%, 2.0% IM vs 1.4% 2G-TKIs, p=0.25) and progression (1.0%, 1.2% IM vs 0.8% 2G-TKIs, p=0.56). Cumulative incidence of discontinuation at 3, 6 and 12 mo. were 5.6%, 10.7% and 19.3%, respectively; values for IM and 2G-TKIs at the three timepoints were 8.1%, 15.0%, 25.5% and 2.5%, 5.3%, 11.5% (p<0.001) (Fig. 1).
Conclusions This real-world study on over 1600 CML patients shows that almost 20% discontinue frontline TKI during the 1st year, mostly for primary resistance or toxicity. Discontinuation rates are higher with IM compared to 2G-TKIs, mostly at 3 mo. and are probably due to a lower attainment of early MR. The impact of older age, higher risks and heavier burden of comorbidities in IM patients should be considered and need deeper investigation.
Elena: GILEAD: Membership on an entity's Board of Directors or advisory committees; NOVARTIS: Membership on an entity's Board of Directors or advisory committees; PFIZER: Membership on an entity's Board of Directors or advisory committees; CELGENE: Other: funding for meeting participation. Sportoletti: AstraZeneca: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria. Stagno: InCyte: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria, Other: Support for attending meetings and/or travel; Novartis: Consultancy, Honoraria, Other: Support for attending meetings and/or travel, Research Funding. Iurlo: Pfizer: Speakers Bureau; Incyte: Speakers Bureau; Novartis: Speakers Bureau; Bristol Myers Squibb: Speakers Bureau. Bonifacio: Novartis: Honoraria; Pfizer: Honoraria; Amgen: Honoraria; Bristol Myers Squibb: Honoraria. Breccia: Pfizer: Honoraria; Incyte: Honoraria; Abbvie: Honoraria; Bristol Myers Squibb/Celgene: Honoraria; Novartis: Honoraria. Latagliata: Novartis: Honoraria; Pfizer: Honoraria; BMS Cellgene: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal